
PIANISSIMO consortium
- Prof. Dr. Maarten Moens (VUB/UZB)
- Prof. Dr. Lisa Goudman (VUB/UZB)
- Prof. Dr. Koen Putman (VUB)
- Prof. Dr. Cleo Crunelle (VUB/UZB)
- Dra. Elke Wuyts (PhD researcher VUB)
- Dra. Frenn Bultinck (PhD researcher VUB)
Study flowchart
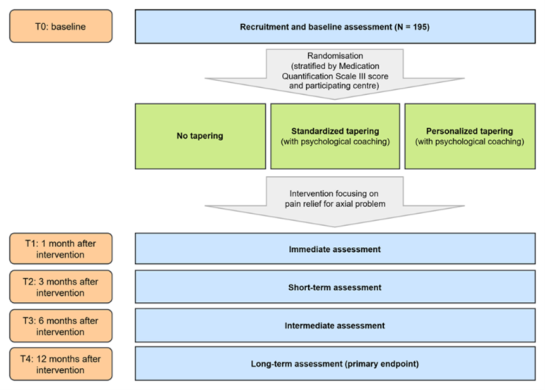
PIANISSIMO consists of a three-arm RCT, whereby two groups will undergo a tapering protocol and one group of patients will receive usual care. In order to make the comparison between patients in the three arms, five assessments will take place: at baseline, and at 1, 3, 6 and 12 months after the treatment focused on pain relief.
Contact
Interested in participating or have questions?
You can always contact our research team, we’ll be happy to help!
You can sign up using the QR code below, and we will get in touch with you:
📧 E-mail: pianissimo@vub.be
📞 Telephone:
- +32 487/48.99.04 (Frenn Bultinck)
- +32 479/02.63.05 (Elke Wuyts)
Project overview
Chronic low back pain is defined as low back pain persisting for at least three months. Patients with chronic low back pain often experience a major impact on their functioning, quality of life, well-being, and medication use. One possible way to help is by providing treatments that focus on pain relief. Such treatments may reduce the need for pain medication, particularly opioids. There is no scientific evidence supporting the long-term use of opioids for chronic non-cancer pain. Moreover, only a limited number of patients are able to stop opioid use completely. In this project, we propose a new treatment strategy: starting with an pain medication tapering program before initiating a pain treatment trajectory for patients with chronic low back pain who use opioids. This approach addresses the high burden of opioid use and aims to create a more logical treatment pathway for a costly and debilitating condition. To evaluate this strategy, we will conduct a three-arm multicenter randomized controlled trial. The study will test whether initiating an opioid tapering program (intervention) before a pain treatment is more effective in reducing disability (primary outcome) after 12 months, compared with usual care (comparison), in patients with chronic low back pain scheduled for a pain treatment. Two different tapering programs will be evaluated. Opioid tapering will be provided either at home or during a hospital stay, depending on the amount of pain medication used. The tapering groups will be compared with a control group of patients who do not undergo opioid tapering before their pain treatment. In addition to disability as the primary outcome, several secondary outcomes will be assessed, including pain intensity, quality of life, participation, domains affected by substance use, anxiety and depression, medication use, and healthcare expenditure. Data will be collected at baseline, and at 1, 3, 6, and 12 months after the pain treatment. |
Study interventions
Usual care
Patients in the usual care group (no tapering protocol before pain treatment) will receive the standard care as currently provided in Belgian practice and hospitals. After the pain treatment, each center can continue their regular care. All interventions will be documented in healthcare utilization diaries.
Standardized tapering protocol
Patients randomized to the standardized tapering protocol will follow a fixed program to discontinue opioids and other pain medications before starting their pain treatment. Depending on the amount of pain medication used, the program will be carried out at home or in hospital. Both at home and in the hospital, all opioids will be discontinued and clonidine (oral or intravenous) will be administered until the end of the program. Paracetamol and diclofenac will also be provided if needed.
Personalized tapering protocol
Patients randomized to the personalized tapering protocol will undergo a program combining opioid substitution and rotation. The goal is to taper opioids before starting the pain treatment. Depending on medication use, this program is provided at home or in hospital.
- Long-acting opioids are first substituted with short-acting opioids.
- Short-acting opioids are then rotated to BuNa (buprenorphine combined with naloxone), under continuous monitoring of withdrawal severity and pain (VAS).
- Withdrawal symptoms are measured with a subjective withdrawal scale (home) or both subjective and objective withdrawal scales (in hospital: Subjective Opioid Withdrawal Scale and Clinical Opioid Withdrawal Scale).
Study status
On behalf of the entire STIMULUS team, we would like to wish you all the best for 2026!
We are starting the year strong. Since last month, 11 new patients have been enrolled in the PIANISSIMO trial, bringing the total number of inclusions to 70. This means that we have already recruited more than one third of the required study population. We are very much looking forward to reaching our next milestone: the inclusion of half of the required population.
Patients were recruited at the AZ Groeninge, AZ Delta, and UZ Brussel study sites.
If you are treating patients with chronic low back pain who are using opioids, we kindly encourage you to refer them to the PIANISSIMO study so that we can support them on their path toward an opioid-free future.
